What is ThyroSeq and how can it help patients?
ThyroSeq® Thyroid Genomic Classifier (GC) is a molecular test specifically designed to determine if a thyroid nodule is benign (not cancer) or malignant (cancer) when cytology result is indeterminate.
ThyroSeq also provides specific information about the genetic makeup of the nodule which allows physicians to determine an individualized course of treatment.
If a ThyroSeq result is negative, the patient can likely avoid surgical removal of their thyroid. If the result is positive, ThyroSeq provides additional information that helps the physician select the most appropriate surgery, which may include preserving part of the patient’s thyroid, protecting natural thyroid function.
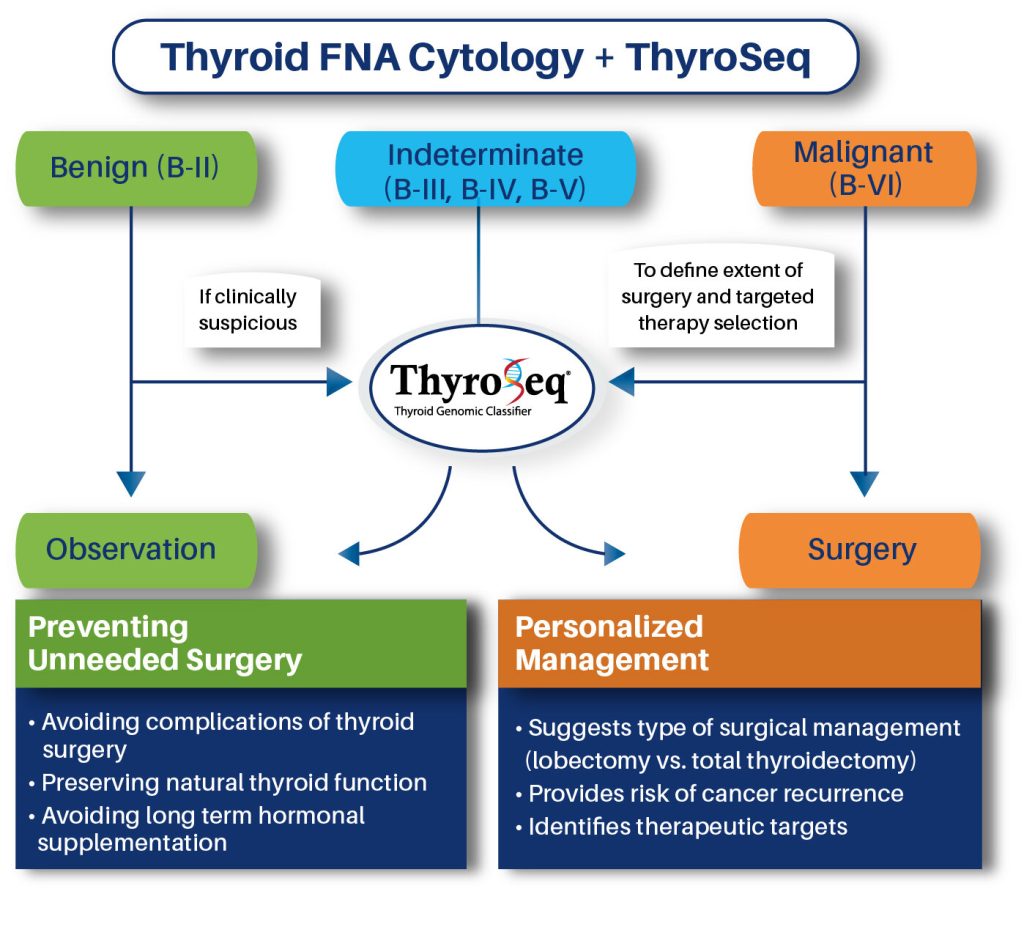
For nodules with malignant cytology (Bethesda VI), ThyroSeq can help inform the extent of surgery and targeted therapy selection. For benign cytology nodules (Bethesda II) with clinical suspicion for malignancy, ThyroSeq may help clarify diagnosis.
Patient Management Using ThyroSeq
ThyroSeq test results refine cancer probability in thyroid nodules with indeterminate cytology, informing the most appropriate management of these patients.
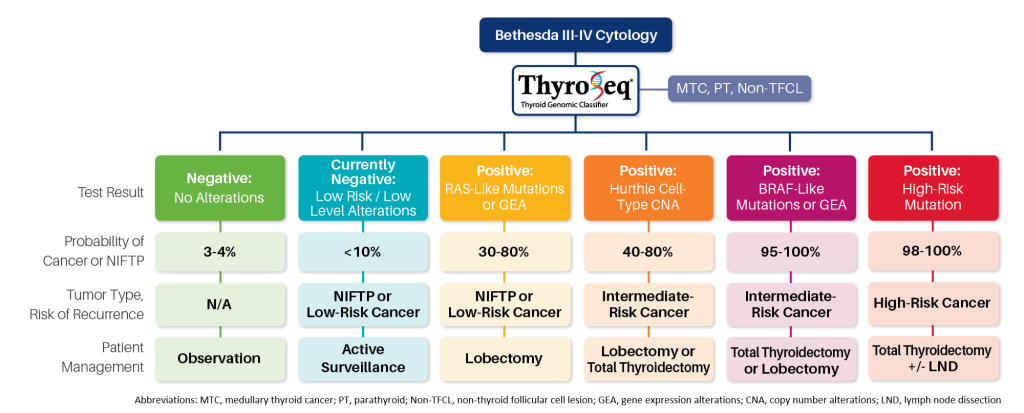
ThyroSeq Utility for Prognostication and Therapy
Comprehensive molecular profiling by ThyroSeq provides preoperative prognostication of cancerous nodules, informing the extent of surgery and therapeutic options.
ThyroSeq provides:
- Detection of clinically actionable therapeutic targets
- Preoperative assessment of the Risk of Cancer Recurrence (RCR)
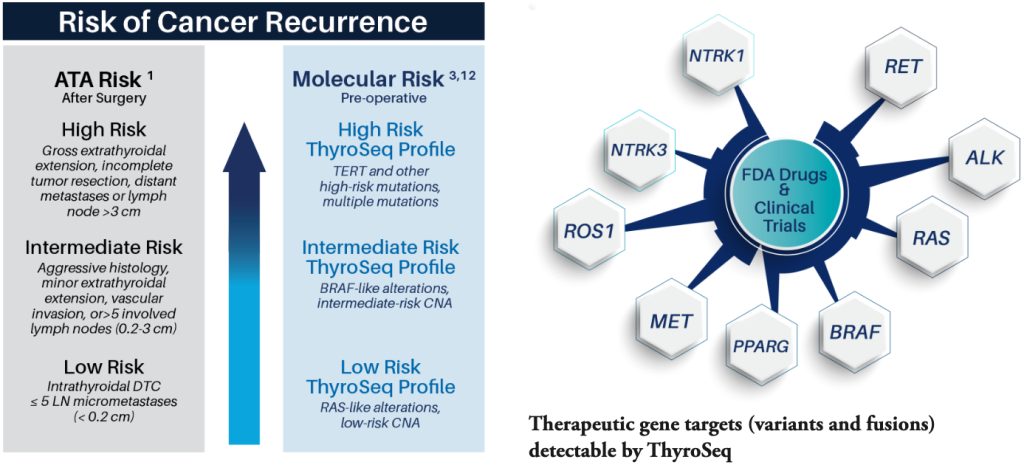
ThyroSeq World-Wide Experience
The ThyroSeq test was designed by expert physicians in the field of thyroid cancer. The test combines years of scientific and clinical experience to help personalize patient care.
- Yang Peiling S, et al. Performance of a multigene genomic classifier and clinical parameters in predicting malignancy in a Southeast Asian cohort of patients with cytologically indeterminate thyroid nodules.
Cancer Cytopathology. 2023. doi: 10.1002/cncy.22796. - Chen T, et al. The Role of the ThyroSeq v3 Molecular Test in the Surgical Management of Thyroid Nodules in the Canadian Public Healthcare Setting. Thyroid. 2020. doi: 10.1089/thy.2019.0539.
- Rajab M, et al. Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada. Cancers. 2022. doi: 10.3390/cancers14174140.
- Hier J, et al. Molecular testing for cytologically suspicious and malignant (Bethesda V and VI) thyroid nodules to optimize the extent of surgical intervention: A retrospective chart review. Journal of Otolaryngology – Head & Neck Surgery. 2021. doi: 10.1186/s40463-021-00500-6
- Lévesque F, et al. Publicly Funded Molecular Testing of Indeterminate Thyroid Nodules: Canada’s Experience. The Journal of Clinical Endocrinology & Metabolism. 2024. doi: 10.1210/clinem/dgae355
How can I order ThyroSeq testing?
ThyroSeq testing is performed on an FNA biopsy, either freshly collected during an FNA procedure or on a previously made FNA cytology smear. Cell block and FFPE surgical tissue slides are also acceptable for ThyroSeq testing.
To set up ThyroSeq testing, please fill out the form. We will get back to you as soon as possible.
I understand that submitting this form, I agree to UPMC’s EU Privacy Notice.
Testimonial
Not accepting this ‘surgery to see’ for my daughter I came to discover ThyroSeq, a totally non-surgical solution in determining just what my daughter’s thyroid nodule was presenting. Following the test and assured by the most impeccable and flawless attention to us as clients, we received the best ever news that my daughter’s nodule was in fact Negative...the same result that a Thyroid Surgeon would have found had a partial Thyroidectomy been needlessly undertaken. We had prepared for a worse outcome but even then we were assured that ThyroSeq’s finding would have guided our own surgeon to perform a more targeted surgery if so needed.
To all at UPMC I cannot thank you enough for this truly impressive and indispensable tool called ThyroSeq. I urge all those presented with an indeterminate thyroid nodule result to not accept this ‘surgery to see’ and instead have your health ‘secured by science’ as ThyroSeq has so plainly proven for my daughter who has been spared the trauma of this standard surgical practice, the possibility of being medicated on in to the future and possible evidence of an operational scar following needless surgery.
— Father of an international ThyroSeq patient

